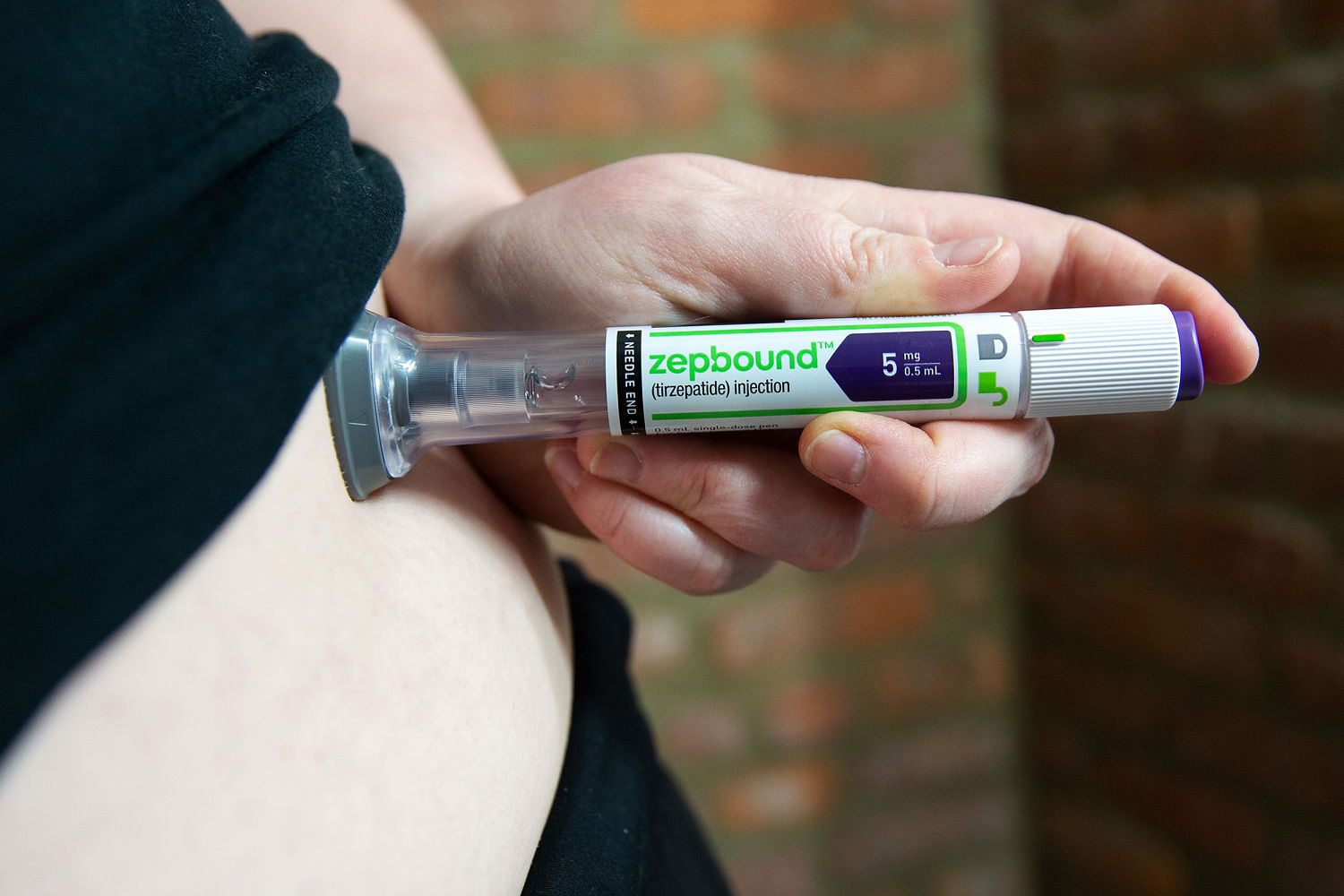
People took Eli Lilly's obesity medication, Zepbough, and lost nearly 50% of their weight, compared to Wegovy, who used rival Novo Nordisk, who used nearly 50% in the first head-to-head study of blockbuster drugs.
Participants in the clinical trial taking Tirzepatide, which sold for Zepbough, lost an average of 50 pounds (22.8 kg) over 72 weeks, while those taking Semaglutide or Wegovy lost about 33 pounds (15 kg). This is based on a research funded by Eli Lilly, published on Sunday in the New England Journal of Medicine.
Both drugs are part of the new type of drugs that regulate appetite and fullness by mimicking hormones in the gut and brain. Dr. Louis Aronne, director of the Weill Cornell Medicine comprehensive weight control center, said Tirzepatide targets two such hormones, namely GLP-1 and GIP, while Semaglutide targets only GLP-1.
"Two drugs together can produce better weight loss," Aronne led the study and presented the findings at the Spanish European Obesity Conference on Sunday.
Although Tirzepatide has won many in Aronne's view of many as "playing competitions," both are important tools for treating obesity, which affects about 40% of American adults.
“The purpose of these drugs is to improve health,” he said. “Most people don’t need the most effective drugs.”
The trial included 751 people from across the United States who were overweight or obese and at least one weight-related health problem, but diabetes was not associated. Participants received the highest dose of Zepbound, 10 mg or 15 mg or Wegovy, 1.7 mg or 2.4 mg each week.
By the end of the trial, those who took Zepbough lost about 20% of their weight on average, while those who took Wegovy lost nearly 14%. The Tirzepatide group has a waist of about 7 inches (17.8 cm), while the semaglutide is about 5 inches (12.7 cm). Additionally, the study found that nearly 32% of people taking Zepbough lost at least a quarter of their weight, while about 16% of people taking Wegovy.
The authors noted that men lost about 6% less weight than women in both groups. As participants in both groups gained weight, they saw improvements in health markers such as blood pressure, blood lipids, and blood sugar levels.
Up to three-quarters of patients taking both medications reported at least one side effect, mainly mild to moderate gastrointestinal problems such as nausea, constipation, diarrhea and vomiting. About 6% of participants left the trial due to adverse events, while 8% of participants taking semaglutide.
According to a survey by KFF, an independent health policy research organization, GLP-1 drugs have become increasingly popular, with at least one in eight U.S. adults reported using their use. Last year, Zepbound's global sales were $4.9 billion. Wegovy brought in nearly $8.8 billion (Denmark Kroner $5.82 billion).
The widespread use of access and affordability is limited. Recently, the U.S. Food and Drug Administration removed tirzepatide and semaglutide from the drug shortage list. Both manufacturers recently released plans that reduce costs to about $500 per month or less based on doses.
Other factors may affect access. CVS Health said that as of July 1, Wegovy will be the first choice for its standard formula or drug list. Zepbough will be excluded.
Dr. Angela Fitch, chief medical officer at obesity company Newwell, said it is important to have a range of drugs that see the disease as widespread. She noted that Wegovy has been found to reduce the risk of severe heart problems by 20%. The medication may work for one patient, but it works well for others.
“We will need to use them just because we have a lot of patients who need treatment,” she added.